- (a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to...(Solved)
(a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to comprise of magnesium Oxide and Magnesium nitride
(i) Write an equation for the reaction leading to formation of the magnesium nitride
(ii) A little water was added to the products in the Petri dish. State and explain the observation made.
(iii) A piece of blue litmus paper was dipped into the solution formed in (b) above.
State the observation made.
Date posted: October 14, 2019. Answers (1)
- A compound can be represented as(Solved)
A compound can be represented as
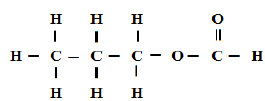
[a] What name is given to the above class of compounds.
[b] Name two reagents that can be reacted together to generate the above compound.
[c] State two conditions necessary for the reaction leading to formation of the above compound to occur.
Date posted: October 14, 2019. Answers (1)
- Chlorine is commonly used in the manufacture of Ca (OCl)2
[i] State one use of the above compound of chlorine
ii] Write a chemical equation leading to...(Solved)
Chlorine is commonly used in the manufacture of Ca (OCl)2
[i] State one use of the above compound of chlorine
ii] Write a chemical equation leading to the production of Ca (OCl)2
Date posted: October 14, 2019. Answers (1)
- In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed...(Solved)
In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed the mixture.
[i]State one observation made.
[ii]Write a chemical equation of the reaction that occurred.
Date posted: October 14, 2019. Answers (1)
- The set-up below was used to investigate properties of the components of air:(Solved)
The set-up below was used to investigate properties of the components of air:
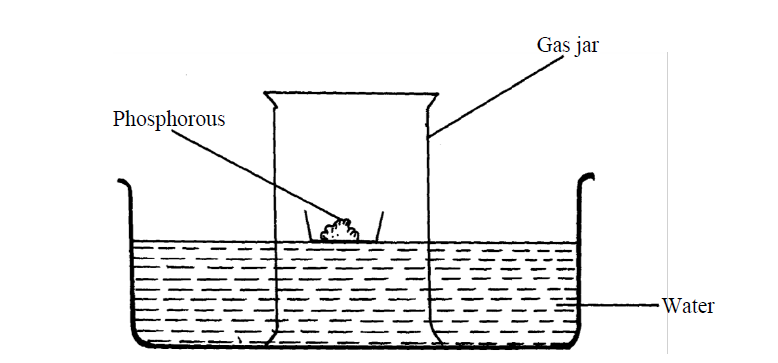
(i) State two observations made during the experiment
(ii) Write two chemical equations for the reactions which occurred
(iii) The experiment was repeated using burning magnesium in place of phosphorous.
There was greater rise of water than in the first case. Explain this observation
(iv) After the two experiments, the water in each trough was tested using blue and red litmus
papers. State and explain the observations of each case.
(a) Phosphorous experiment
b) magnesium experiment
(v) Briefly explain how a sample of nitrogen gas can be isolated from air in the laboratory
Date posted: October 14, 2019. Answers (1)
- An hydrocarbon can be represented as: C2 H2(Solved)
An hydrocarbon can be represented as: C2 H2
[a] Name the hydrocarbon.
[b] State two reagents that can be reacted together to generate the hydrocarbon.
[c] Identify the group of hydrocarbons into which C2 H2 belongs to.
Date posted: October 14, 2019. Answers (1)
- The reduction potentials of elements M and N are:(Solved)
The reduction potentials of elements M and N are:
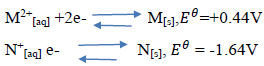
Using the above reduction potentials, predict whether a reaction would occur between
N+[aq] and M[[s]
Date posted: October 14, 2019. Answers (1)
- Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide(Solved)
Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide
Date posted: October 14, 2019. Answers (1)
- The table shows the colours obtained when some indicators are added to solutions:-(Solved)
The table shows the colours obtained when some indicators are added to solutions:-

(a) Complete the table by filling in the missing colours
(b) Identify indicator W
Date posted: October 14, 2019. Answers (1)
- In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What...(Solved)
In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What is meant by the term alkali?
(b) Explain using physical properties of the gas why ammonia is not collected by downward delivery
Date posted: October 14, 2019. Answers (1)
- The following data gives the pH values of some solutions(Solved)
The following data gives the pH values of some solutions

(a) What colour change would occur in solution P on addition of two drops of phenolphthalein indicator?
(b) State the pH value of a resulting solution when equal moles of solution P and R react
Date posted: October 14, 2019. Answers (1)
- Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate(Solved)
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
Date posted: October 14, 2019. Answers (1)
- Use the information given below to answer the questions that follow:(Solved)
Use the information given below to answer the questions that follow:
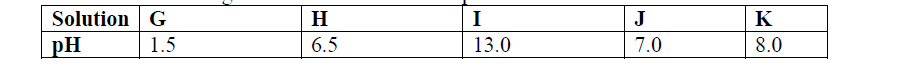
(a) Which of the solutions would be used to relieve a stomach upset caused by indigestion?
(b) Which solution is likely to be:
(i) Dilute sulphuric acid?
(ii) Sodium hydroxide solution?
Date posted: October 14, 2019. Answers (1)
- Complete the table below to show the colour of the given indicator in acidic and basic solutions.(Solved)
a) Complete the table below to show the colour of the given indicator in acidic and basic solutions.
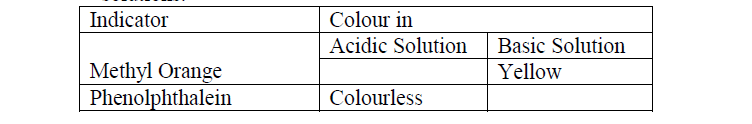
b) How does the PH value of 0.1M potassium hydroxide solution compare with that of 0.1M
aqueous ammonia? Explain.
Date posted: October 14, 2019. Answers (1)
- Below are the pH values of 4 types of medicine represented by letters P, Q, R and S(Solved)
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
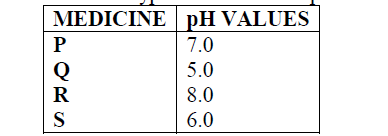
a) It is not advisable to use S when a patient has indigestion .Explain
b) What is the role of chemistry in drug manufacture
Date posted: October 11, 2019. Answers (1)
- a)Name two particles likely to be emitted when a radioactive nuclide undergoes radioactivity.
b)The half-life of a radioactive nuclide is 3 hours. Given that its initial...(Solved)
a)Name two particles likely to be emitted when a radioactive nuclide undergoes radioactivity.
b)The half-life of a radioactive nuclide is 3 hours. Given that its initial mass is 288g, determine the remaining mass after 12 hours.
Date posted: October 11, 2019. Answers (1)
- During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30...(Solved)
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes.
[a] Write the ionic equation of the reaction that occurred at the cathode.
b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode.
Date posted: October 11, 2019. Answers (1)
- A reaction can be represented as;(Solved)
A reaction can be represented as;
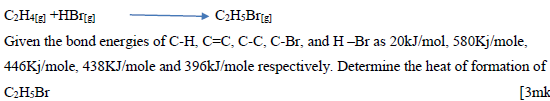
Date posted: October 11, 2019. Answers (1)
- A polymer can be represented as follows(Solved)
A polymer can be represented as follows

[a] Name and draw the structure of the monomer.
[b] What type of polymerization occurs in the above case?
[c] Given that the molecular mass of the polymer is 25620, how many units of the monomer make the polymer.
Date posted: October 11, 2019. Answers (1)
- At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.(Solved)
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
Date posted: October 11, 2019. Answers (1)